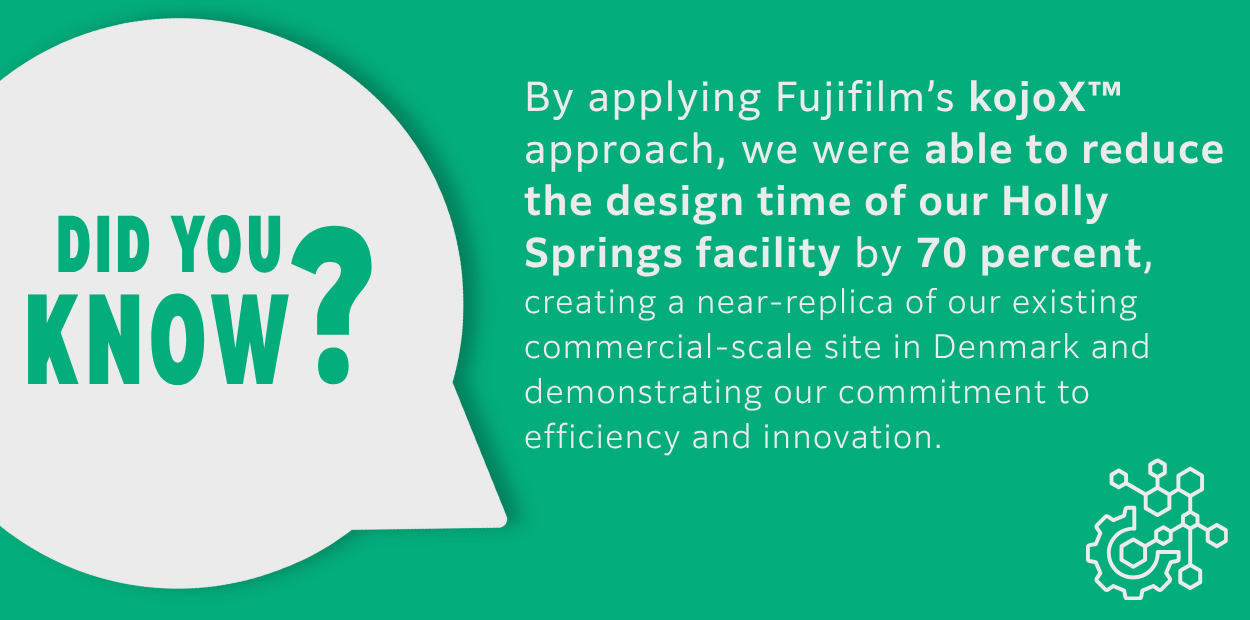
October 2025 Newsletter
October 2025 Newsletter FUJIFILM Biotechnologies is proud to be an innovative CDMO partner, seamlessly guiding our customers though their journey of creating life-impacting medicines from initial process development stages through to full commercial supply. Catch up on our latest news and insights below.
|
|---|
Lars Petersen named ‘CEO of the Year’ We are thrilled to announce that Lars Petersen won ‘CEO of the Year’ this week at the CPHI Pharma Awards 2025, in recognition of his transformational leadership within the CDMO industry.
|
|---|
SOLUTIONS SPOTLIGHT Process Development |
|---|
Looking to develop or optimize your process? Explore our process development services | Learn how in silico strategies can enhance efficiency in monoclonal antibody bioprocess development | We can partner with you to develop a suitable formulation for your drug substance or drug product |
|---|
NOW TRENDING In the News Catch up on some of the most recent stories:
|
|---|
Pharma Manufacturing Webinar November 11 | 4 pm GMT James Pullen, Senior Director of Downstream Process Development, will join a panel discussion on Optimizing Purification Strategies for Non-Traditional mAbs. |
|---|
Xtalks Webinar November 17 | 11 am EST Ian Brown, Senior Director, PC & PV Sciences, will be presenting on Using Acceleration Levers to Expedite Late Phase CMC Programs for Therapeutic Proteins. |
|---|
Fierce Biotech Webinar In Case You Missed It Steve Loftus, Director, Commercial Technical Lead – Biologics, gave an engaging webinar on October 14 on Shaping the Future of Microbial Development: From Gene to GMP. |
|---|